2025-11-06
Global Stage, New Chapter: Sen

Canagliflozin, a new sodium–glucose cotransporter 2 inhibitor, in the treatment of diabetes

Release Time: 2020-10-12

News Source: SenovaTech

Author: Operation Dept.
API information:
Product name:Canpagliflozin Cas No.:842133-18-0
Molecular Formula:C24H25FO5S Molecular Weight:444.51600
Structure:
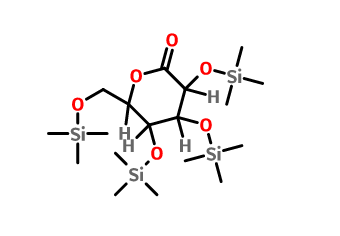
Key intermediates information:
Product name:
(3R,4S,5R,6R)-3,4,5-tris(triMethylsilyloxy)-6-((triMethylsilyloxy)Methyl)tetrahydro-2H-pyran-2-one
Cas No.:32384-65-9
Molecular Formula:C18H42O6Si4 Molecular Weight:466.86448
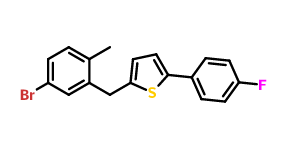
Structure:
Product name:2-(5-BroMo-2-Methylbenzyl)-5-(4-fluorophenyl)thiophene
Cas No.:1030825-20-7
Molecular Formula:C18H14BrFS Molecular Weight:361.2711632
Structure:
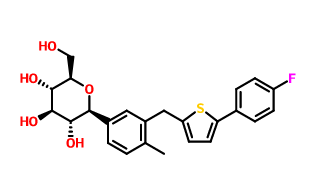
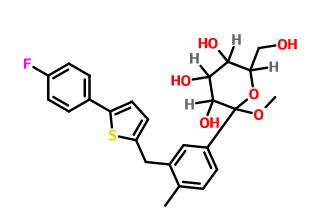
Product name:
D-Glucopyranoside,Methyl 1-C-[3-[[5-(4-fluorophenyl)-2-thienyl]Methyl]-4-Methylphenyl]-
Cas No.:1030825-21-8
Molecular Formula:C25H27FO6S Molecular Weight:474.5416832
Structure:
Product name:
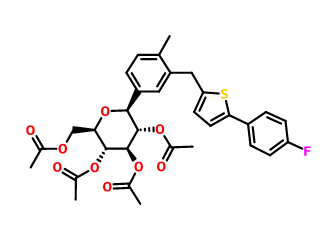
D-Glucitol, 1,5-anhydro-1-C-[3-[[5-(4-fluorophenyl)-2-thienyl]Methyl]-4-Methylphenyl]-, tetraacetate, (1S)- (9CI)
Cas No.:866607-35-4
Molecular Formula:C32H33FO9S Molecular Weight:612.6624232
Structure:
.................................................................................................................................................................................................
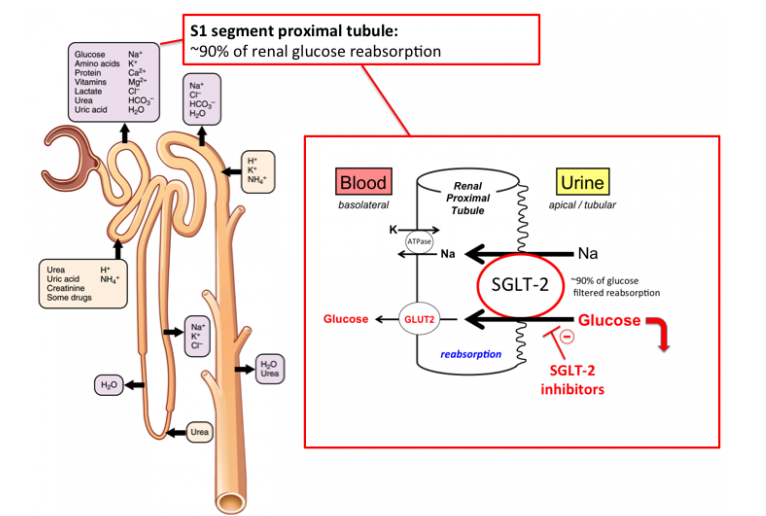
Canagliflozin belongs to a class of agents—the sodium–glucose co-transporter 2 (SGLT2) inhibitors—whose novel mechanism of action offers potential advantages over other antihyperglycemic agents, including a relatively low hypoglycemia risk and weight loss-promoting effects. Canagliflozin has dose-dependent pharmacokinetics, and research in laboratory animals demonstrated high oral bioavailability (85%) and rapid effects in lowering glycosylated hemoglobin (HbA1c) values. In four early-stage clinical trials involving a total of over 500 patients, the use of canagliflozin for varying periods was associated with significant mean reductions in HbA1c (absolute reductions of 0.45–0.92%) and fasting plasma glucose (decreases ranged from 16.2% to 42.4%) and weight loss ranging from 0.7 to 3.5 kg.
.................................................................................................................................................................................................
As Anti-diabetes series drug,Canagliflozin is similar as its brother drug-Empagliflozin.Canagliflozin and Empagliflozin can reduce the incidence of major cardiovascular events and have a good protective effect on the kidney.
The capacity of Canpagliflozin API is 600-800kg/month with Facility GMP and DMF is being working.
Headquarter Office in ShenZhen, R&D center in HangZhou, GMP factory in JiangSu.
(Please note:All products which might have patent infringment are used for R&D only/ It's Not Senova's responsibility in those markets where there are patent infringement regulated.)
PLANT:


WORKSHOP:


R&D ROOM:

WAREHOUSE:

PACKING:

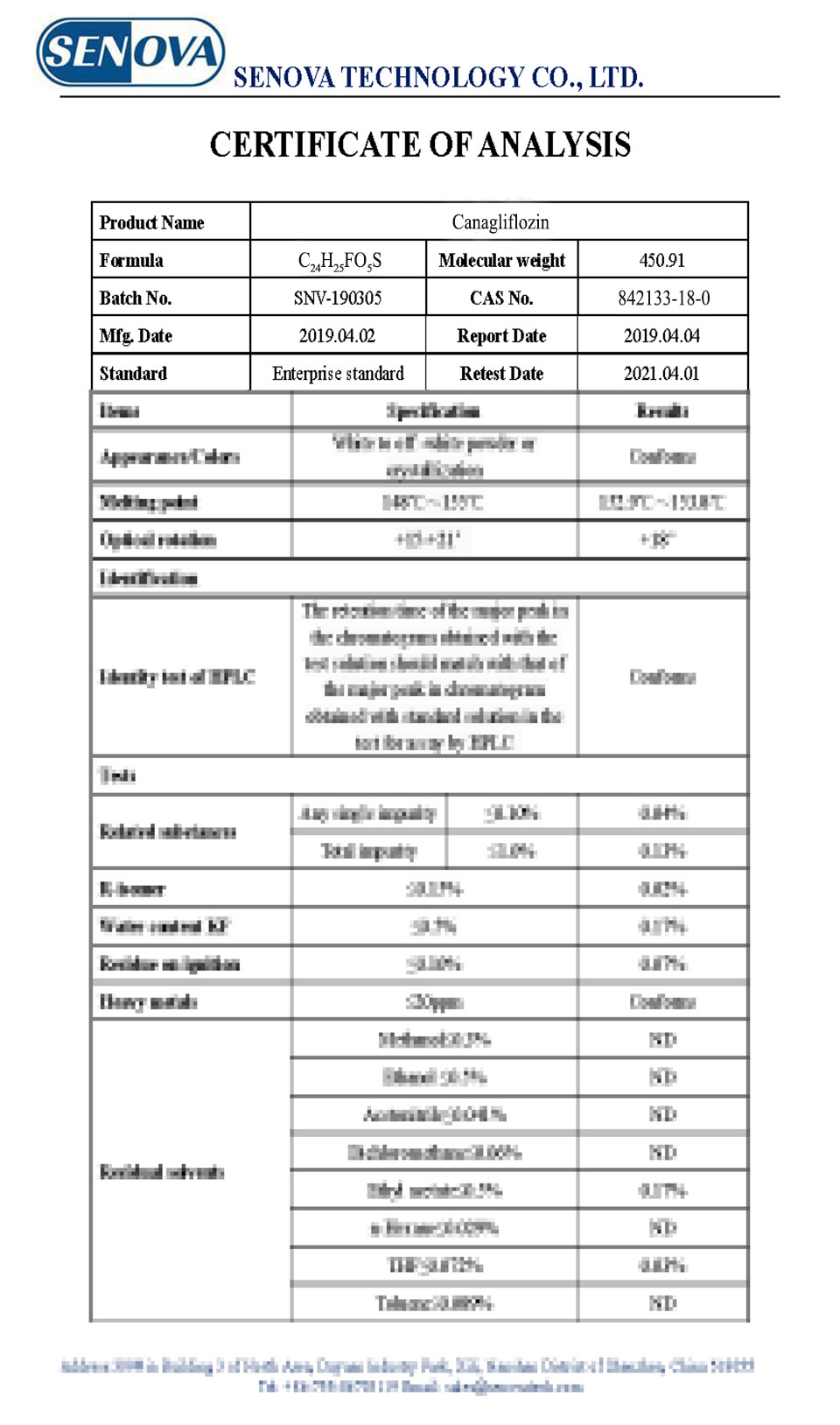
COA:
.................................................................................................................................................................................................
SENOVA:
Senova means“Step For Innovation", we are a professional company who's supplying APIs(mainly New Generics), Advanced Intermediates and KSM(Key Starting Materials) as well as technical Support/Registration support/Custom synthesis service in pharmaceutical and related field , we focus on serving those manufacturers' New Generics Projects from R&D stage to Commercial Stage from meterials to documents support(DMF/CMC). For those regulated markets, we secure all of our products can be produced under GMP regulation with Full Documentation.