2025-11-06
Global Stage, New Chapter: Sen

Release Time: 2024-09-06

News Source: SenovaTech

Author: SENOVA—Lucas
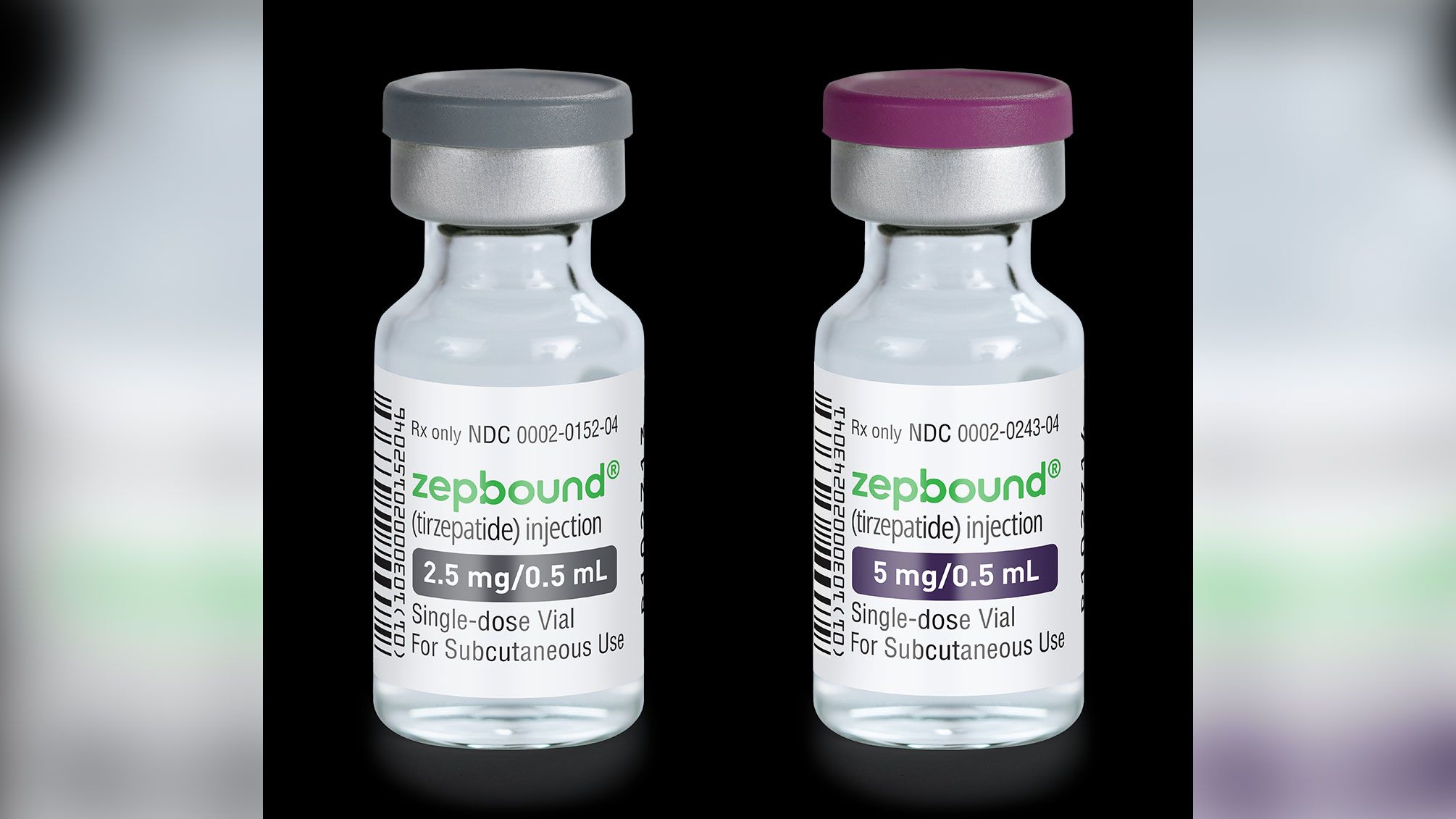
Tirzepatide GLP-1/GIPR dual agonist commercial product name Mounjaro (type 2 diabetes) Zepbound (obesity)
Mounjaro and Zepbound both contain the same active ingredient, tirzepatide and are made by Eli Lilly and Company. They are both available as single-dose pens in the same strengths 2.5 mg, 5 mg, 7.5 mg, 10 mg, 12.5 mg,15 mg per 0.5 mL.Normally, when we eat, natural hormones called GIP and GLP-1 are released by the gut. These hormones increase insulin release, suppress appetite, slow gastric emptying, and increase the feeling of fullness. Tirzeptide works like our natural hormones GIP and GLP-1 by activating the GIP and GLP-1 receptors.
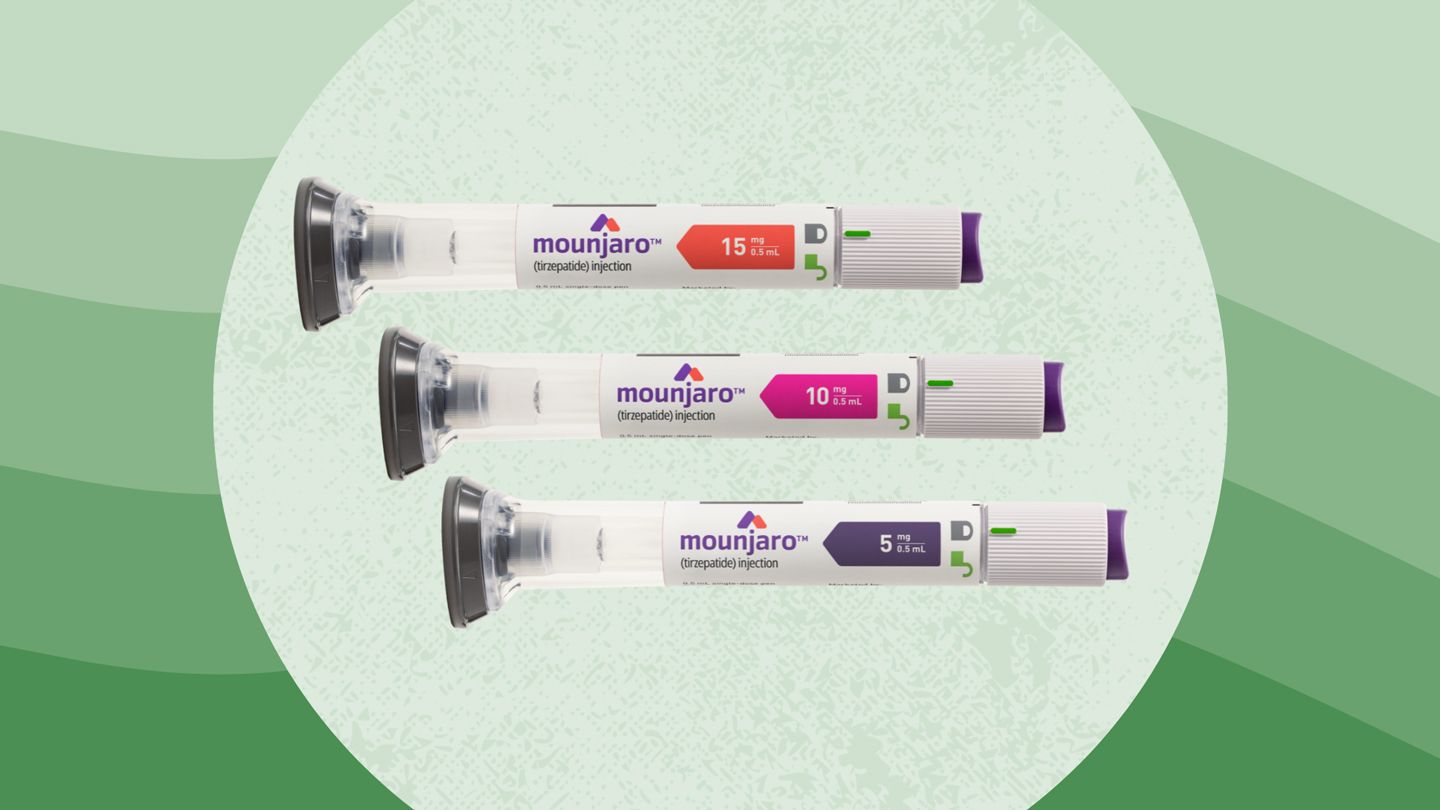
Active ingredient: tirzepatide
Mounjaro Inactive ingredients: sodium chloride, sodium phosphate dibasic heptahydrate, and water for injection. Hydrochloric acid solution and/or sodium hydroxide solution may have been added to adjust the pH.
Zepbound Inactive ingredients: sodium chloride, sodium phosphate dibasic heptahydrate, and water for injection. Hydrochloric acid solution and/or sodium hydroxide solution may have been added to adjust the pH.
Effect
Patients on Zepbound (tirzepatide) 5mg weekly lost 16.1 kg (35.5 lb) on average after 72 weeks. For Zepbound's 10mg weekly dose, the average weight loss was 22.2 kg (48.9 lb), and for Zepbound's 15mg weekly dose average weight loss was 23.6 kg (52.0 lb) over 72 weeks. Patients who used a placebo lost 2.4 kg (5.3 lb) over the 72 weeks. These results are from the clinical trial SURMOUNT-1, NCT04184622. Individual results may vary.
Side Effects
The most common tirzepatide side effects include abdominal pain, burping, constipation, diarrhea, dyspepsia, fatigue, gastroesophageal reflux disease, hair loss, hypersensitivity reactions, injection site reactions, nausea, and vomiting, which affects 5% or more patients.
Tirzepatide Dosing information
Usual Zepbound Dose for Weight Loss (Adult)
Initial dose: 2.5 mg under the skin (subcutaneously) once a week.
After 4 weeks: The dosage should be increased to 5 mg subcutaneously once a week.
Further dose increases: After at least 4 weeks on the current dose the dosage may be increased in 2.5 mg increments.
Recommended maintenance dose: 5 mg, 10 mg, or 15 mg injected subcutaneously once weekly.
Maximum dose: 15 mg subcutaneously once a week.
Usual Mounjaro Dose for Diabetes Type 2 (Adult)
Initial dose: 2.5 mg under the skin (subcutaneously) once a week.
After 4 weeks: The dosage should be increased to 5 mg subcutaneously once a week.
If additional glycemic control is needed: The dosage should be increased in 2.5 mg increments after at least 4 weeks on the current dose.
Maximum dose: 15 mg subcutaneously once a week.
Comments: The 2.5 mg dosage is for starting of treatment and is not intended for glycemic control. The day of weekly administration can be changed, if necessary, as long as the time between the 2 doses is at least 3 days (72 hours).
Storage
Store in the refrigerator between 36⁰F to 46⁰F (2⁰C to 8⁰C).
Do not freeze. Do not use if frozen.
Store the single-dose pens in the original carton until use to protect them from light.
If needed, each single-dose pen can be stored at room temperature up to 86⁰F (30⁰C) for up to 21 days.