2025-11-06
Global Stage, New Chapter: Sen

Release Time: 2020-09-11

News Source: SenovaTech

Author: Operation Dept.
Product information:
Product name:Empagliflozin Cas No.:864070-44-0
Molecular Formula:C23H27ClO7 Molecular Weight:450.90928
Structure:
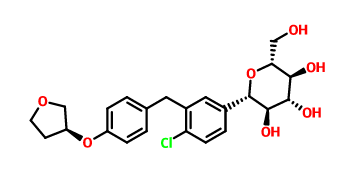
Key intermediates information:
Product name:(3S)-3-[4-[(5-bromo-2-chlorophenyl)methyl]phenoxy]oxolane
Cas No.:915095-89-5
Molecular Formula:C17H16BrClO2 Molecular Weight:367.66500
Structure:
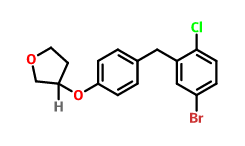
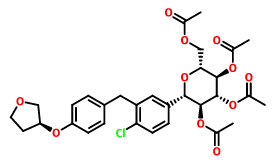
Product name:(1S)-1,5-anhydro-2,3,4,6-tetra-O-acteyl-1-C-[4-chloro-3-[[4-[[(3S)
-tetrahydrofu-ran-3-yl]oxy]phenyl] methyl]phenyl]-D-Glucitol
Cas No.:915095-99-7
Molecular Formula:C31H35ClO11 Molecular Weight:619.056
Structure:
..............................................................................................................................
Empagliflozin, sold under the brand name Jardiance among others, is a medication used together with diet and exercise to treat type 2 diabetes.
Empagliflozin is used in combination with proper diet and exercise to help people with type 2 diabetes lower their blood sugar levels. It can be used alongside other medications for type 2 diabetes such as metformin, sulfonylureas, and insulin. When compared to a placebo, empagliflozin led to a drop of 0.7% in hemoglobin A1c, a long-term marker of blood glucose levels.
............................................................................................................
The number of people with diabetes rose from 108 million in 1980 to 422 million in 2014.SENOVE started to study Empagliflozin API in 2015, and it only took two years from small trial to medium trial to commercialization.The capacity of EMPAGLIFLOZIN API is 1500kg/month with Facility GMP and DMF.The capacity of the key intermediates(N-1&N-2) are 1000-3000kg/month now.
Headquarter Office in ShenZhen, R&D center in HangZhou, GMP factory in JiangSu.
(Please note:All products which might have patent infringment are used for R&D only/ It's Not Senova's responsibility in those markets where there are patent infringement regulated.)
PLANT:


WORKSHOP


R&D ROOM:

WAREHOUSE:

PACKING:

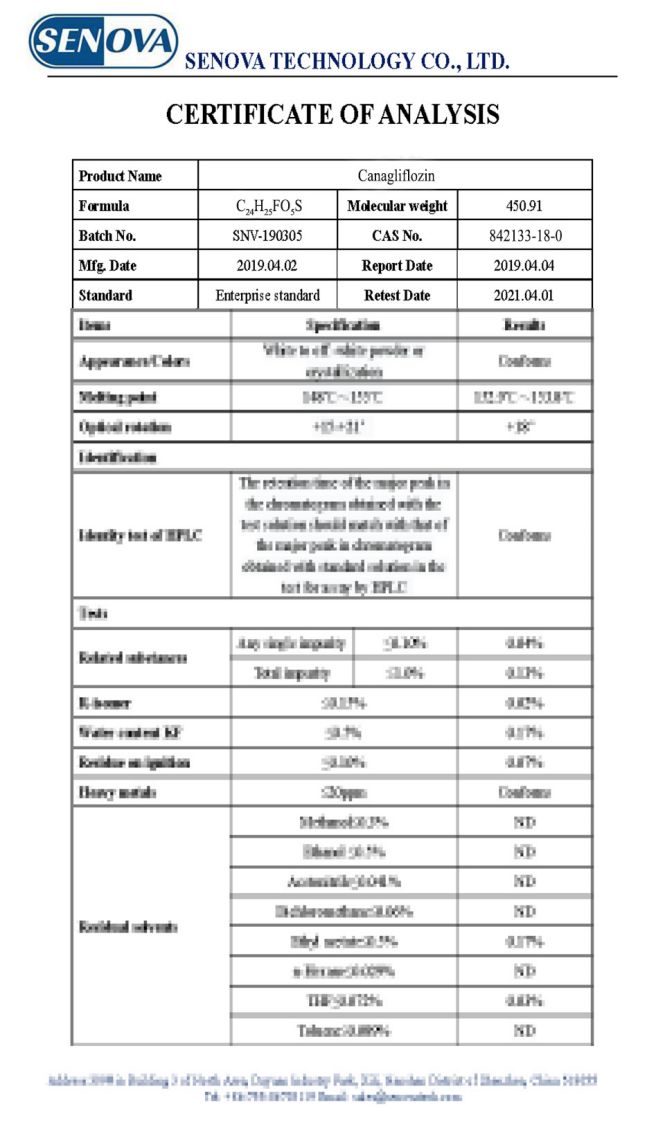
COA:
.................................................................................................................................
SENOVA:
Senova means“Step For Innovation", we are a professional company who's supplying APIs(mainly New Generics), Advanced Intermediates and KSM(Key Starting Materials) as well as technical Support/Registration support/Custom synthesis service in pharmaceutical and related field , we focus on serving those manufacturers' New Generics Projects from R&D stage to Commercial Stage from meterials to documents support(DMF/CMC). For those regulated markets, we secure all of our products can be produced under GMP regulation with Full Documentation.